Education
- Exceptions To Vsepr Theory
Valence Shell Electron Pair Repulsion theory does not always predict the correct geometry of molecules. Examples of exceptions include: transition metal molecules (e.g., CrO3 is trigonal bipyramidal, TiCl4 is tetrahedral)odd-electron molecules (CH3 is...
- Vsepr Theory
Valence Shell Electron Pair Repulsion (VSPER) theory is used to predict the geometric shape of the molecules based on the electron repulsive force. There are some limitation to VSEPR.IntroductionThe shapes of the molecules is determined mainly by the...
- Formation Of H2o Molecule(bonds)
H2O molecule contains two Hydrogen atoms linked with a Oxygen atom. So we can describe the formation of Water molecule bond either by valence shell electron pair repultion theory or by Hybridization concept. ...
- Most Important Entrance Questions For Mbbs
The study of plants related to chemistry is:a) Pteridology b) Sericulturec) Biochemistry d) PhytochemistryTobacco mosaic virus (TMV) genes are:a) Double standard RNA b) Single standard RNA c) Polyribonucleotides d) Double standard DNASexual...
- Discovery Of Protons
Protons are subatomic particles that, with neutrons and electrons, are the principal constituents of atoms. Protons are positively charged atoms that reside in the nucleus of an atom. These protons add the overall positive charge of a molecule. The mass...
Education
Double and Triple Bonds in VSEPR Theory
Molecular geometry is determined by possible locations of an electron in a valence shell, not by how many how many pairs of valence electrons are present.
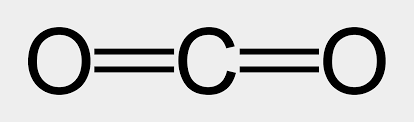 To see how the model works for a molecule with double bonds, consider carbon dioxide, CO2. Carbon has four pairs of bonding electrons, there are only two places electrons can be found in this molecule (in each of the double bonds with oxygen). Repulsion between the electrons is least when the double bonds are on opposite sides of the carbon atom. This forms a linear molecule that has a 180° bond angle.
To see how the model works for a molecule with double bonds, consider carbon dioxide, CO2. Carbon has four pairs of bonding electrons, there are only two places electrons can be found in this molecule (in each of the double bonds with oxygen). Repulsion between the electrons is least when the double bonds are on opposite sides of the carbon atom. This forms a linear molecule that has a 180° bond angle.
For another example, consider the carbonate ion, CO32-.
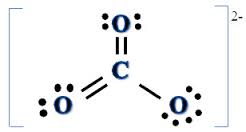
As with carbon dioxide, there are four pairs of valence electrons around the central carbon atom. Two pairs are in single bonds with oxygen atoms, while two pairs are part of a double bond with an oxygen atom. This means there are three locations for electrons. Repulsion between electrons is minimized when the oxygen atoms form an equilateral triangle around the carbon atom. Therefore, VSEPR theory predicts the carbonate ion will take a trigonal planar shape, with a 120° bond angle.
For another example, consider the carbonate ion, CO32-.
As with carbon dioxide, there are four pairs of valence electrons around the central carbon atom. Two pairs are in single bonds with oxygen atoms, while two pairs are part of a double bond with an oxygen atom. This means there are three locations for electrons. Repulsion between electrons is minimized when the oxygen atoms form an equilateral triangle around the carbon atom. Therefore, VSEPR theory predicts the carbonate ion will take a trigonal planar shape, with a 120° bond angle.
- Exceptions To Vsepr Theory
Valence Shell Electron Pair Repulsion theory does not always predict the correct geometry of molecules. Examples of exceptions include: transition metal molecules (e.g., CrO3 is trigonal bipyramidal, TiCl4 is tetrahedral)odd-electron molecules (CH3 is...
- Vsepr Theory
Valence Shell Electron Pair Repulsion (VSPER) theory is used to predict the geometric shape of the molecules based on the electron repulsive force. There are some limitation to VSEPR.IntroductionThe shapes of the molecules is determined mainly by the...
- Formation Of H2o Molecule(bonds)
H2O molecule contains two Hydrogen atoms linked with a Oxygen atom. So we can describe the formation of Water molecule bond either by valence shell electron pair repultion theory or by Hybridization concept. ...
- Most Important Entrance Questions For Mbbs
The study of plants related to chemistry is:a) Pteridology b) Sericulturec) Biochemistry d) PhytochemistryTobacco mosaic virus (TMV) genes are:a) Double standard RNA b) Single standard RNA c) Polyribonucleotides d) Double standard DNASexual...
- Discovery Of Protons
Protons are subatomic particles that, with neutrons and electrons, are the principal constituents of atoms. Protons are positively charged atoms that reside in the nucleus of an atom. These protons add the overall positive charge of a molecule. The mass...
