Education
- Double And Triple Bonds In Vsepr Theory
Molecular geometry is determined by possible locations of an electron in a valence shell, not by how many how many pairs of valence electrons are present. To see how the model works for a molecule with double bonds, consider...
- Exceptions To Vsepr Theory
Valence Shell Electron Pair Repulsion theory does not always predict the correct geometry of molecules. Examples of exceptions include: transition metal molecules (e.g., CrO3 is trigonal bipyramidal, TiCl4 is tetrahedral)odd-electron molecules (CH3 is...
- Predicting The Shapes Of Molecules
There is no direct relationship between the formula of a compound and the shape of its molecules. The shapes of these molecules can be predicted from their Lewis structures, however, with a model developed about 30 years ago, known as the valence-shellelectron-pair...
- Concepts Of Acids And Bases
Acids and bases have been given several concepts so that we can recognize them.Mainly Three concepts have been given.They are: 1)Arrhenius concept =>This concept states that,"A acid is a species which gives [H^+] ion in an aqueous solution and base...
- Discovery Of Protons
Protons are subatomic particles that, with neutrons and electrons, are the principal constituents of atoms. Protons are positively charged atoms that reside in the nucleus of an atom. These protons add the overall positive charge of a molecule. The mass...
Education
Formation Of H2O Molecule(Bonds)
H2O molecule contains two Hydrogen atoms linked with a Oxygen atom. So we can describe the formation of Water molecule bond either by valence shell electron pair repultion theory or by Hybridization concept.
Hybridization concept is more reliable so I explain with it here.
In H2O molecule , the central atom Oxygen undergoes sp^3 Hybridization forming four identical hybrid orbitals. But there are only two H- atoms available to get linked or bonded so two H- atoms are bonded with two hybrid O- orbitals.
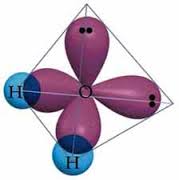
Now there are two more orbitals (hybrid) which are not bonded.
We can apply here Valance Shell Electron Pair Repultion Theory.According to this theory ,there occurs repultion between two bond pair and two lone pair electrons to be more stable. Due to this lone pair-bond pair repultion the expected geometry tetrahedral is distorted to triangular planner structure making an angle 104.5 degree with central O Oxygen atom.
In this way H2O bond or single H2O molecule is formed.
Hybridization concept is more reliable so I explain with it here.
In H2O molecule , the central atom Oxygen undergoes sp^3 Hybridization forming four identical hybrid orbitals. But there are only two H- atoms available to get linked or bonded so two H- atoms are bonded with two hybrid O- orbitals.
Now there are two more orbitals (hybrid) which are not bonded.
We can apply here Valance Shell Electron Pair Repultion Theory.According to this theory ,there occurs repultion between two bond pair and two lone pair electrons to be more stable. Due to this lone pair-bond pair repultion the expected geometry tetrahedral is distorted to triangular planner structure making an angle 104.5 degree with central O Oxygen atom.
In this way H2O bond or single H2O molecule is formed.
- Double And Triple Bonds In Vsepr Theory
Molecular geometry is determined by possible locations of an electron in a valence shell, not by how many how many pairs of valence electrons are present. To see how the model works for a molecule with double bonds, consider...
- Exceptions To Vsepr Theory
Valence Shell Electron Pair Repulsion theory does not always predict the correct geometry of molecules. Examples of exceptions include: transition metal molecules (e.g., CrO3 is trigonal bipyramidal, TiCl4 is tetrahedral)odd-electron molecules (CH3 is...
- Predicting The Shapes Of Molecules
There is no direct relationship between the formula of a compound and the shape of its molecules. The shapes of these molecules can be predicted from their Lewis structures, however, with a model developed about 30 years ago, known as the valence-shellelectron-pair...
- Concepts Of Acids And Bases
Acids and bases have been given several concepts so that we can recognize them.Mainly Three concepts have been given.They are: 1)Arrhenius concept =>This concept states that,"A acid is a species which gives [H^+] ion in an aqueous solution and base...
- Discovery Of Protons
Protons are subatomic particles that, with neutrons and electrons, are the principal constituents of atoms. Protons are positively charged atoms that reside in the nucleus of an atom. These protons add the overall positive charge of a molecule. The mass...
