Education
- Exceptions To Vsepr Theory
Valence Shell Electron Pair Repulsion theory does not always predict the correct geometry of molecules. Examples of exceptions include: transition metal molecules (e.g., CrO3 is trigonal bipyramidal, TiCl4 is tetrahedral)odd-electron molecules (CH3 is...
- Vsepr Theory
Valence Shell Electron Pair Repulsion (VSPER) theory is used to predict the geometric shape of the molecules based on the electron repulsive force. There are some limitation to VSEPR.IntroductionThe shapes of the molecules is determined mainly by the...
- Formation Of H2o Molecule(bonds)
H2O molecule contains two Hydrogen atoms linked with a Oxygen atom. So we can describe the formation of Water molecule bond either by valence shell electron pair repultion theory or by Hybridization concept. ...
- Best Chemistry Syllabus For Ioe Entrance Examination
CHEMISTRY SYLLABUS FOR IOE ENTRANCEUnit I. Language of Chemistry & Physical Chemistry:Symbol, formulate valency and chemical questionsProblems based on chemical equations (relation with weight and weight, and weight and volume);Atomic Structure: Study...
- Discovery Of Protons
Protons are subatomic particles that, with neutrons and electrons, are the principal constituents of atoms. Protons are positively charged atoms that reside in the nucleus of an atom. These protons add the overall positive charge of a molecule. The mass...
Education
Predicting the Shapes of Molecules
There is no direct relationship between the formula of a compound and the shape of its molecules. The shapes of these molecules can be predicted from their Lewis structures, however, with a model developed about 30 years ago, known as the valence-shellelectron-pair repulsion (VSEPR) theory.
The VSEPR theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence shell of that atom. The five compounds shown in the figure below can be used to demonstrate how the VSEPR theory can be applied to simple molecules.
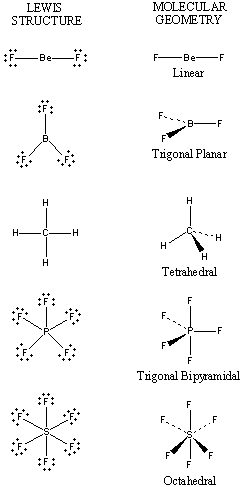
The VSEPR theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence shell of that atom. The five compounds shown in the figure below can be used to demonstrate how the VSEPR theory can be applied to simple molecules.

- There are only two places in the valence shell of the central atom in BeF2 where electrons can be found. Repulsion between these pairs of electrons can be minimized by arranging them so that they point in opposite directions. Thus, the VSEPR theory predicts that BeF2 should be a linear molecule, with a 180o angle between the two Be-F bonds.
- There are three places on the central atom in boron trifluoride (BF3) where valence electrons can be found. Repulsion between these electrons can be minimized by arranging them toward the corners of an equilateral triangle. The VSEPR theory therefore predicts a trigonal planargeometry for the BF3 molecule, with a F-B-F bond angle of 120o.
- BeF2 and BF3 are both two-dimensional molecules, in which the atoms lie in the same plane. If we place the same restriction on methane (CH4), we would get a square-planar geometry in which the H-C-H bond angle is 90o. If we let this system expand into three dimensions, however, we end up with a tetrahedral molecule in which the H-C-H bond angle is 109o28'.
- Repulsion between the five pairs of valence electrons on the phosphorus atom in PF5 can be minimized by distributing these electrons toward the corners of a trigonal bipyramidal. Three of the positions in a trigonal bipyramidal are labeled equatorial because they lie along the equator of the molecule. The other two are axial because they lie along an axis perpendicular to the equatorial plane. The angle between the three equatorial positions is 120o, while the angle between an axial and an equatorial position is 90o.
- There are six places on the central atom in SF6where valence electrons can be found. The repulsion between these electrons can be minimized by distributing them toward the corners of an octahedron. The term octahedronliterally means "eight sides," but it is the six corners, or vertices, that interest us. To imagine the geometry of an SF6 molecule, locate fluorine atoms on opposite sides of the sulfur atom along the X, Y, and Zaxes of an XYZ coordinate system.
- Exceptions To Vsepr Theory
Valence Shell Electron Pair Repulsion theory does not always predict the correct geometry of molecules. Examples of exceptions include: transition metal molecules (e.g., CrO3 is trigonal bipyramidal, TiCl4 is tetrahedral)odd-electron molecules (CH3 is...
- Vsepr Theory
Valence Shell Electron Pair Repulsion (VSPER) theory is used to predict the geometric shape of the molecules based on the electron repulsive force. There are some limitation to VSEPR.IntroductionThe shapes of the molecules is determined mainly by the...
- Formation Of H2o Molecule(bonds)
H2O molecule contains two Hydrogen atoms linked with a Oxygen atom. So we can describe the formation of Water molecule bond either by valence shell electron pair repultion theory or by Hybridization concept. ...
- Best Chemistry Syllabus For Ioe Entrance Examination
CHEMISTRY SYLLABUS FOR IOE ENTRANCEUnit I. Language of Chemistry & Physical Chemistry:Symbol, formulate valency and chemical questionsProblems based on chemical equations (relation with weight and weight, and weight and volume);Atomic Structure: Study...
- Discovery Of Protons
Protons are subatomic particles that, with neutrons and electrons, are the principal constituents of atoms. Protons are positively charged atoms that reside in the nucleus of an atom. These protons add the overall positive charge of a molecule. The mass...
