Education
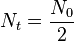
- Laws Of Radioactive Decay
The Radioactive Decay Law Let us now use some symbols to reduce the amount of writing we have to do to describe what is going on and to avail ourselves of some mathematical techniques to simplify the situation...
- New Horizons Reveals More Mountains In Pluto's Heart
The frozen peaks were found on the lower-left edge of the dwarf world's "heart" and are 1-1.5km-high.They sit between a patch of icy, flat terrain, called Sputnik Planum, which scientists believe is less than 100 million years old, and a dark area...
- Egyptian Doctor In 1300 Bc
The ancient Egyptians suffered numerous epidemics and often tomb art described epidemics and parasitic infestation such as hydatidosis. Egyptians medicine reached a very high standard, and its reputation spread to neighboring countries. A student doctor...
- Dr. Von Mering And Paracetamol
Paracetamol had been synthesized in 1877 at Johns Hopkins University in Baltimore. It had no evidence medical value and thereafter had simply sat on a shelf for sixteen years. In 1893, P-hydroxy-acetanilide was evaluated clinically by Dr. von Mering (1849-1908),...
- History Of Cereal Milling
The purpose of flour milling is to isolate the starchy endosperm in as pure a state as possible, uncontaminated by other germ or bran. The process of flour milling dates back to Egyptian and earlier times. There are illustrations from ancient inscriptions...
Education
Half Life Of Radio Active Decay
Most of us have not been taught to think instinctively in terms of logarithmic or exponential terms even though many natural phenomena display exponential behaviours. Most of the forms of thinking which we have been taught in school are based on linear changes and as a result it is rather difficult for us to grasp the Radioactive Decay Law intuitively. For this reason an indicator is usually derived from the law which helps us think more clearly about what is going on.
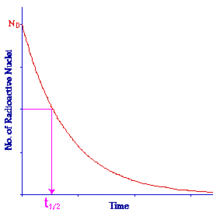
This indicator is called the Half Life and it expresses the length of time it takes for the radioactivity of a radioisotope to decrease by a factor of two. From a graphical point of view we can say that when:
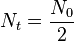
the time taken is the Half Life:

Note that the half-life does not express how long a material will remain radioactive but simply the length of time for its radioactivity to halve. Examples of the half lives of some radioisotopes are given in the following table. Notice that some of these have a relatively short half life. These tend to be the ones used for medical diagnostic purposes because they do not remain radioactive for very long following administration to a patient and hence result in a relatively low radiation dose.
| Radioisotope | Half Life (approx.) |
|---|---|
| 81mKr | 13 seconds |
| 99mTc | 6 hours |
| 131I | 8 days |
| 51Cr | 1 month |
| 137Cs | 30 years |
| 241Am | 462 years |
| 226Ra | 1620 years |
| 238U | 4.51 x 109 years |
But they do present a logistical problem when we wish to use them when there may not be a radioisotope production facility nearby. For example suppose we wish to use 99mTc for a patient study and the nearest nuclear facility for making this isotope is 5,000 km away. The production facility could be in Sydney and the patient could be in Perth for instance. After making the isotope at the nuclear plant it would be decaying with a half life of 6 hours. So we put the material on a truck and drive it to Sydney airport. The isotope would be decaying as the truck sits in Sydney traffic then decaying still more as it waits for a plane to take it to Perth. Then decaying more as it is flown across to Perth and so on. By the time it gets to our patient it will have substantially reduced in radioactivity possibly to the point of being useless for the patient's investigation. And what about the problem if we needed to use 81mKr instead of 99mTc for our patient? You will see in another chapter of this book that logistical challenges such as this have given rise to quite innovative solutions. More about that later!
You can appreciate from the table above that other isotopes have a very long half lives. For example 226Ra has a half life of over 1,500 years. This isotope has been used in the past for therapeutic applications in medicine. Think about the logistical problems here. They obviously do not relate to transporting the material from the point of production to the point of use. But they relate to how the material is kept following its arrival at the point of use. We must have a storage facility so that the material can be kept safely for a long period of time. But for how long? A general rule of thumb for the quantities of radioactivity used in medicine is that the radioactivity will remain significant for about 10 half lives. So we would have to have a safe environment for storage of the 226Ra for about 16,000 years! This storage facility would have to be secure from many unforeseeable events such as earthquakes, bombing etc. and be kept in a manner which our children's, children's children can understand. A very serious undertaking indeed!
See
See
RELATIONSHIP BETWEEN THE DECAY CONSTANT AND THE HALF LIFE
- Laws Of Radioactive Decay
The Radioactive Decay Law Let us now use some symbols to reduce the amount of writing we have to do to describe what is going on and to avail ourselves of some mathematical techniques to simplify the situation...
- New Horizons Reveals More Mountains In Pluto's Heart
The frozen peaks were found on the lower-left edge of the dwarf world's "heart" and are 1-1.5km-high.They sit between a patch of icy, flat terrain, called Sputnik Planum, which scientists believe is less than 100 million years old, and a dark area...
- Egyptian Doctor In 1300 Bc
The ancient Egyptians suffered numerous epidemics and often tomb art described epidemics and parasitic infestation such as hydatidosis. Egyptians medicine reached a very high standard, and its reputation spread to neighboring countries. A student doctor...
- Dr. Von Mering And Paracetamol
Paracetamol had been synthesized in 1877 at Johns Hopkins University in Baltimore. It had no evidence medical value and thereafter had simply sat on a shelf for sixteen years. In 1893, P-hydroxy-acetanilide was evaluated clinically by Dr. von Mering (1849-1908),...
- History Of Cereal Milling
The purpose of flour milling is to isolate the starchy endosperm in as pure a state as possible, uncontaminated by other germ or bran. The process of flour milling dates back to Egyptian and earlier times. There are illustrations from ancient inscriptions...
