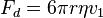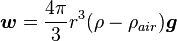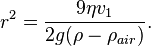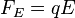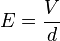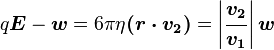Education
- Was Einstein The First To Invent E = Mc2?
In present world, no equation is more famous than E = mc2, and few are simpler. Indeed, the immortal equation’s fame rests largely on that utter simplicity: the energy E of a system is equal to its mass m multiplied by c2, the speed of light squared....
- What Is General Relativity?
Theory Of General Relativity:- General relativity is a theory of gravitation developed by Einstein in the years 1907–1915. The development of general relativity began with the equivalence principle, under which the states...
- Main Properties Of Cathode Rays
Do you know how electrons were discovered?If not click here PROPERTIES OF CATHODE RAYSORIGIN These rays originate from cathode.PATH Cathode rays travel in straight line.PRESSURE Cathode rays exert mechanical pressure.SHADOW FORMATION The...
- Hseb Possible Physics Questions Of Class 11
PHYSICS | QUESTION MODEL HSEB QUESTION MODEL OF PHYSICS WITH POSSIBLE QUESTIONS CLASS : 11 For : 2070 HSEB Model Question Time : 3 hrsFull Marks : 75Pass marks : 27 Group ‘A’Q.1. Answer in brief, any SIX questions. [2 X 6 = 12] ...
- Millikan, Robert Andrews (1868-1953)
US physicist, who was awarded the 1923 Nobel prize for Physics for his determination of the charge of a single electron and for validating Albert Einstein photoelectric equation. He also made important contribution to American science as an educator,...
Education
Millikan's Oil drop experiment

The oil drop experiment was an experiment performed by Robert A. Millikan and Harvey Fletcher in 1909 to measure the elementary electric charge (the charge of the electron).
The experiment entailed observing tiny charged droplets of oil between two horizontal metal electrodes. First, with zero applied electric field, the terminal velocity of a droplet was measured. At terminal velocity, the drag force equals the gravitational force, and these depend on the radius in different ways, so that the radius of the droplet, and therefore the mass and gravitational force, could be determined (using the known density of the oil). Then an adjustable voltage was applied between the plates to induce an electric field, and the voltage was adjusted until the drops were suspended in mechanical equilibrium, indicating that the electrical force and the gravitational force were balanced. Now using the known electric field, Millikan and Fletcher could determine the charge on the oil droplet. By repeating the experiment for many droplets, they confirmed that the charges were all small integer multiples of a certain base value, which was found to be 1.5924(17)×10−19 C, within 1% of the currently accepted value of1.602176487(40)×10−19 C. They proposed that this was the (negative of the) charge of a single electron.
Experimental procedure
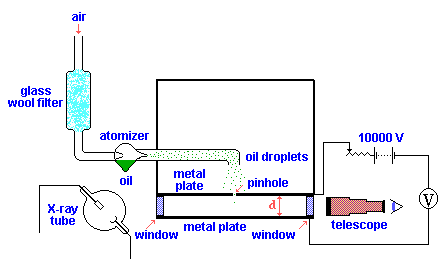
Millikan’s and Fletcher's apparatus incorporated a parallel pair of horizontal metal plates. By applying a potential difference across the plates, a uniform electric field was created in the space between them. A ring of insulating material was used to hold the plates apart. Four holes were cut into the ring, three for illumination by a bright light, and another to allow viewing through a microscope.A fine mist of oil droplets was sprayed into a chamber above the plates. The oil was of a type usually used invacuum apparatus and was chosen because it had an extremely low vapour pressure. Ordinary oil would evaporate under the heat of the light source causing the mass of the oil drop to change over the course of the experiment. Some oil drops became electrically charged through friction with the nozzle as they were sprayed. Alternatively, charging could be brought about by including an ionising radiation source (such as an X-ray tube). The droplets entered the space between the plates and, because they were charged, could be made to rise and fall by changing the voltage across the plates.
Method
Initially the oil drops are allowed to fall between the plates with the electric field turned off. They very quickly reach aterminal velocity because of friction with the air in the chamber. The field is then turned on and, if it is large enough, some of the drops (the charged ones) will start to rise. (This is because the upwards electric force FE is greater for them than the downwards gravitational force Fg, in the same way bits of paper can be picked by a charged rubber rod). A likely looking drop is selected and kept in the middle of the field of view by alternately switching off the voltage until all the other drops have fallen. The experiment is then continued with this one drop.
The drop is allowed to fall and its terminal velocity v1 in the absence of an electric field is calculated. The drag force acting on the drop can then be worked out using Stokes' law:
where v1 is the terminal velocity (i.e. velocity in the absence of an electric field) of the falling drop, η is the viscosityof the air, and r is the radius of the drop.
The weight w is the volume D multiplied by the density ρ and the acceleration due to gravity g. However, what is needed is the apparent weight. The apparent weight in air is the true weight minus the upthrust (which equals the weight of air displaced by the oil drop). For a perfectly spherical droplet the apparent weight can be written as:
At terminal velocity the oil drop is not accelerating. Therefore the total force acting on it must be zero and the two forces F and w must cancel one another out (that is, F = w). This implies
Once r is calculated, w can easily be worked out.
Now the field is turned back on, and the electric force on the drop is
where q is the charge on the oil drop and E is the electric field between the plates. For parallel plates
where V is the potential difference and d is the distance between the plates.
One conceivable way to work out q would be to adjust V until the oil drop remained steady. Then we could equate FE with w. Also, determining FE proves difficult because the mass of the oil drop is difficult to determine without reverting to the use of Stokes' Law. A more practical approach is to turn V up slightly so that the oil drop rises with a new terminal velocity v2. Then
- Was Einstein The First To Invent E = Mc2?
In present world, no equation is more famous than E = mc2, and few are simpler. Indeed, the immortal equation’s fame rests largely on that utter simplicity: the energy E of a system is equal to its mass m multiplied by c2, the speed of light squared....
- What Is General Relativity?
Theory Of General Relativity:- General relativity is a theory of gravitation developed by Einstein in the years 1907–1915. The development of general relativity began with the equivalence principle, under which the states...
- Main Properties Of Cathode Rays
Do you know how electrons were discovered?If not click here PROPERTIES OF CATHODE RAYSORIGIN These rays originate from cathode.PATH Cathode rays travel in straight line.PRESSURE Cathode rays exert mechanical pressure.SHADOW FORMATION The...
- Hseb Possible Physics Questions Of Class 11
PHYSICS | QUESTION MODEL HSEB QUESTION MODEL OF PHYSICS WITH POSSIBLE QUESTIONS CLASS : 11 For : 2070 HSEB Model Question Time : 3 hrsFull Marks : 75Pass marks : 27 Group ‘A’Q.1. Answer in brief, any SIX questions. [2 X 6 = 12] ...
- Millikan, Robert Andrews (1868-1953)
US physicist, who was awarded the 1923 Nobel prize for Physics for his determination of the charge of a single electron and for validating Albert Einstein photoelectric equation. He also made important contribution to American science as an educator,...